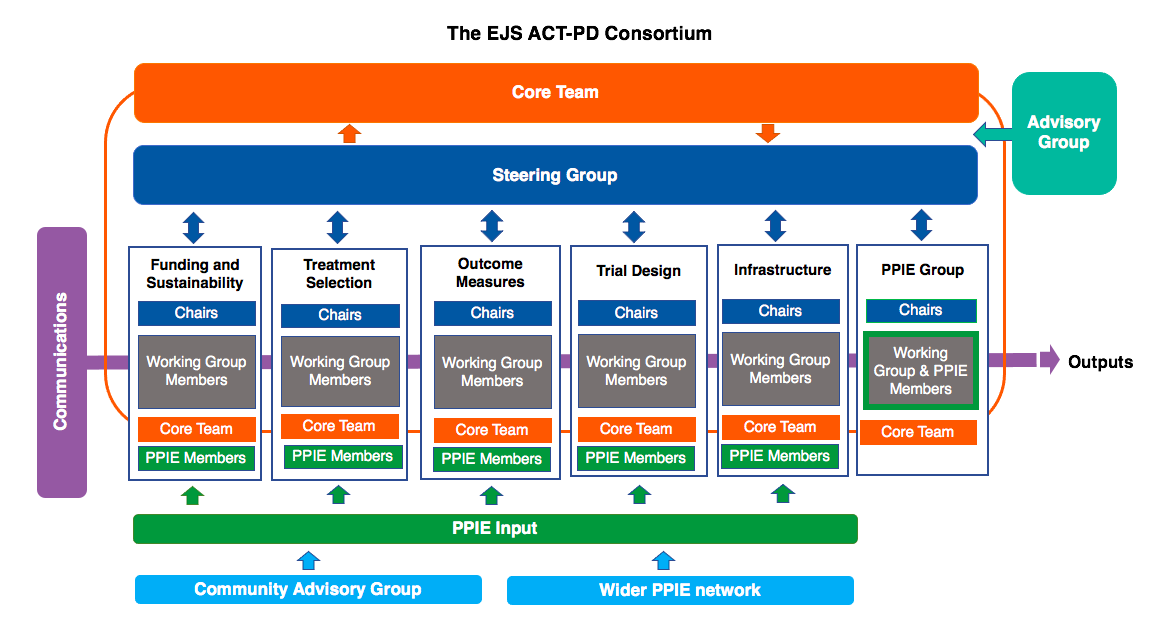
Project Structure
We have assembled 6 expert groups to focus on the different decisions required to produce the trial:
- Trial Design
- Treatment Selection
- Outcome Measures
- Infrastructure
- Funding & Sustainability
- Patient and Public Involvement and Engagement
The groups are made up of a range of Parkinson’s stakeholders including Clinicians, Trial Methodologists, Statisticians, Health Economists, Clinical Trials Pharmacists, Charities, and Funders. Most importantly, at least 2 patient or care partner representatives are members of each group, to ensure a focus on patient-needs is maintained in all discussions. Each Working Group has also each been assigned a Clinical Research Fellow and Administrative Assistant from the Core Management team to help facilitate their progress and collaboration.
The EJS ACT-PD Initiative provides a unique opportunity to contribute towards developing the next generation of clinical academic researchers. We therefore underwent a national recruitment process to select a team of Early Career Researcher’s to join our Working Groups and contribute to the sustainability of the platform.
The groups present their progress and decisions to the Steering Group at regular intervals for feedback and approval.
The project also benefits from our International Advisory Group of key senior researchers who provide invaluable guidance and advice from an international perspective.
Project Overview
The EJS ACT-PD Consortium is comprised of 6 Working Groups, an overarching Steering Group and an International Advisory Group. Whilst each Working Group is focused on a specific element of the project, they work collaboratively to reach consensus on all decisions made.